Mixed-sequence pyrrolidine-amide oligonucleotide mimics: Boc(Z) synthesis and DNA/RNA binding properties†
Abstract
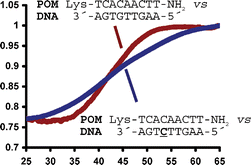
Pyrrolidine-amide oligonucleotide mimics (POMs) exhibit promising properties for potential applications, including in vivo DNA and RNA targeting, diagnostics and bioanalysis. Before POMs can be evaluated in these applications it is first necessary to synthesise and establish the properties of fully modified oligomers, with biologically relevant mixed sequences. Accordingly, Boc-Z-protected thyminyl, adeninyl and cytosinyl POM monomers were prepared and used in the first successful solid phase synthesis of a mixed sequence POM, Lys-TCACAACTT-NH2. UV thermal denaturation studies revealed that the POM oligomer is capable of hybridising with sequence selectivity to both complementary parallel and antiparallel RNA and DNA strands. Whilst the duplex melting temperatures (Tm) were higher than the corresponding duplexes formed with isosequential PNA, DNA and RNA oligomers the rates of association/dissociation of the mixed sequence POM with DNA/RNA targets were noticeably slower.

 Please wait while we load your content...
Please wait while we load your content...