Design and synthesis of orally active dispiro 1,2,4,5-tetraoxanes; synthetic antimalarials with superior activity to artemisinin†‡
Abstract
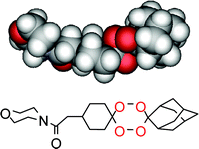
Unsymmetrical dispiro- and spirotetraoxanes have been designed and synthesized via acid-catalyzed cyclocondensation of bis(hydroperoxides) with

 Please wait while we load your content...
Please wait while we load your content...