Synthesis and Claisen rearrangement of bridged bicyclic enol ethers of relevance to the course of ketene s-cis-diene cycloaddition
Abstract
The synthesis is described of a range of 3-alkylidene-2-oxabicyclo[2.2.1]hept-5-ene and 3-alkylidene-2-oxabicyclo[2.2.2]oct-5-ene derivatives;
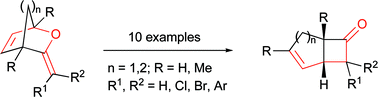

 Please wait while we load your content...
Please wait while we load your content...