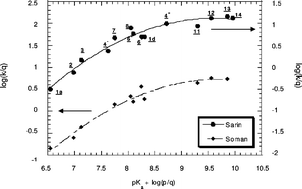
Following a potentiometric determination of the relevant pKa values of the (R1R2)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH functionality, the second order rate constants (kOx) for reaction of a large set of oximate bases with two model organophosphorus esters, i.e. bis-(4-nitrophenyl)phenylphosphonate (BNPPP) and bis-(4-nitrophenyl)methylphosphonate (BNPMP), and three toxic compounds, i.e., sarin (GB), soman (GD) and diisopropylphosphorofluoridate (DFP), in aqueous as well as a 30 : 70 (v/v) H2O–Me2SO mixture have been measured. The corresponding Brønsted-type nucleophilicity plots of log kOxvs. pKaOx reveal a clear tendency of the reactivity of the oximates to suffer a saturation effect with increasing basicity in aqueous solution. In the case of BNPMP and the three toxic esters, this behaviour is reflected in a levelling off at pKa
≈ 9 but a more dramatic situation prevails in the BNPPP system where the attainment of maximum reactivity at pKa
≈ 9 is followed by a clear decrease in rate at higher pKa's. Interestingly, a number of data reported previously by different authors for the sarin, soman and DFP systems are found to conform rather well to the curvilinear Brønsted correlations built with our data. Based on this and previous results obtained for reactions at carbon centers, it can be concluded that the observed saturation effect is the reflection of an intrinsic property of the oximate functionality. An explanation of this behavior in terms of an especially strong requirement for desolvation of the oximates prior to nucleophilic attack which becomes more and more difficult with increasing basicity is suggested. This proposal is supported by the observed changes in pKaOx and kOx brought about by a transfer from H2O to a 30 : 70 H2O–Me2SO mixture. The implications of the saturation effect on the efficiency of oximates as nucleophilic catalysts for smooth decontamination are emphasized. Also discussed is the effect of basicity on the exalted (α-effect) reactivity of these bases.
NOH functionality, the second order rate constants (kOx) for reaction of a large set of oximate bases with two model organophosphorus esters, i.e. bis-(4-nitrophenyl)phenylphosphonate (BNPPP) and bis-(4-nitrophenyl)methylphosphonate (BNPMP), and three toxic compounds, i.e., sarin (GB), soman (GD) and diisopropylphosphorofluoridate (DFP), in aqueous as well as a 30 : 70 (v/v) H2O–Me2SO mixture have been measured. The corresponding Brønsted-type nucleophilicity plots of log kOxvs. pKaOx reveal a clear tendency of the reactivity of the oximates to suffer a saturation effect with increasing basicity in aqueous solution. In the case of BNPMP and the three toxic esters, this behaviour is reflected in a levelling off at pKa
≈ 9 but a more dramatic situation prevails in the BNPPP system where the attainment of maximum reactivity at pKa
≈ 9 is followed by a clear decrease in rate at higher pKa's. Interestingly, a number of data reported previously by different authors for the sarin, soman and DFP systems are found to conform rather well to the curvilinear Brønsted correlations built with our data. Based on this and previous results obtained for reactions at carbon centers, it can be concluded that the observed saturation effect is the reflection of an intrinsic property of the oximate functionality. An explanation of this behavior in terms of an especially strong requirement for desolvation of the oximates prior to nucleophilic attack which becomes more and more difficult with increasing basicity is suggested. This proposal is supported by the observed changes in pKaOx and kOx brought about by a transfer from H2O to a 30 : 70 H2O–Me2SO mixture. The implications of the saturation effect on the efficiency of oximates as nucleophilic catalysts for smooth decontamination are emphasized. Also discussed is the effect of basicity on the exalted (α-effect) reactivity of these bases.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH functionality, the second order rate constants (kOx) for reaction of a large set of oximate bases with two model organophosphorus
NOH functionality, the second order rate constants (kOx) for reaction of a large set of oximate bases with two model organophosphorus 

 Please wait while we load your content...
Please wait while we load your content...