Asymmetric synthesis of cyclopropanes and dihydrofurans based on phosphine oxide chemistry†
Abstract
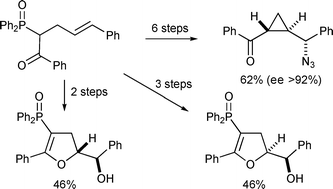
The asymmetric synthesis of γ-azido trans-cyclopropyl ketones is accomplished via a short, simple and efficient sequence. The cyclopropanation step is achieved by an intramolecular nucleophilic ring closure, with a diphenylphosphinate leaving group, to give

 Please wait while we load your content...
Please wait while we load your content...