Chavicol formation in sweet basil (Ocimum basilicum): cleavage of an esterified C9 hydroxyl group with NAD(P)H-dependent reduction
Abstract
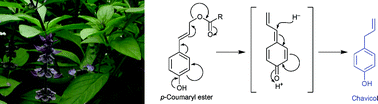
Propenyl- and allyl-phenols, such as methylchavicol, p-anol and eugenol, have gained importance as flavoring agents and also as putative precursors in the biosynthesis of 9,9′-deoxygenated lignans, many of which have potential medicinal applications. In spite of several decades of investigation, however, the complete biosynthetic pathway to a propenyl/allylphenol had not yet been reported. We have subjected a Thai basil variety accumulating relatively large amounts of the simplest volatile allylphenol, methylchavicol, to in vivo administration of radiolabeled precursors and assays of protein preparations in vitro. Through these experiments, the biosynthesis of chavicol was shown to occur via the phenylpropanoid pathway to p-coumaryl alcohol. Various possibilities leading to deoxygenation of the latter were examined, including reduction of the side-chain double bond to form p-dihydrocoumaryl alcohol, followed by dehydration to afford chavicol, as well as formation of p-methoxycinnamyl alcohol, with further side-chain modification to afford methylchavicol. A third possibility studied was activation of the side-chain alcohol of p-coumaryl alcohol, e.g.via esterification, to form a more facile leaving group via reductive elimination. The latter was shown to be the case using p-coumaryl esters as potential substrates for a NAD(P)H-dependent reductase to afford chavicol, which is then O-methylated to afford methylchavicol.

 Please wait while we load your content...
Please wait while we load your content...