1,3-Dipolar character of 2-vinyl quinazoline 3-oxides; first and second generation cycloaddition products
Abstract
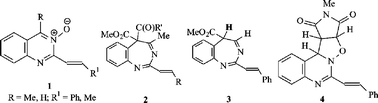
The reaction between the heteroaromatic N-oxides 1a, 1b and 1c with dimethyl acetylenedicarboxylate or methyl propiolate furnishes 1,3-benzodiazepines, the products of ring transformations of primarily formed cycloadducts. The structures of 8a and 10a have been confirmed by X-ray crystallographic analysis. The aldonitrone 1c also reacts with N-methylmaleimide and with phenyl vinyl sulfone to furnish the first examples of primary cycloaddition products from quinazoline 3-oxides.

 Please wait while we load your content...
Please wait while we load your content...