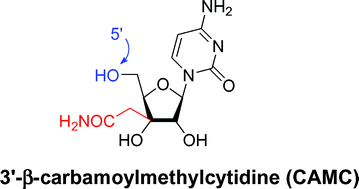
Synthesis of 3′-β-carbamoylmethylcytidine (CAMC) and its derivatives as potential antitumor agents†
Abstract
3′-β-Carbamoylmethylcytidine (CAMC) and its derivatives were synthesized using an intramolecular Reformatsky-type reaction promoted by SmI2 as the key step. In vitro tumor cell growth inhibitory activity was evaluated and CAMC was found to exhibit potent cytotoxicity against various human tumor cell lines. From a structure–activity relationship study it was postulated that the cytotoxic mechanism of action of CAMC did not require phosphorylation at the 5′-hydroxyl group. This study provides a novel strategy for the development of a new type of antitumor

 Please wait while we load your content...
Please wait while we load your content...