Concise synthesis of aryl-C-nucleosides by Friedel–Crafts alkylation
Abstract
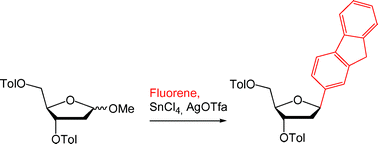
A fast and simplified synthesis of 1′,2′-dideoxy-1′-pyrenyl-riboside and several other C-nucleosides is shown. Shelf-stable 1-O-methyl-3,5-di-O-toluoyl-2-deoxyribose is demonstrated to serve as a versatile glycosyl donor in Lewis acid promoted Friedel–Crafts alkylations of unsubstituted pyrene and other inexpensive arenes such as fluorene and methylnaphthalene. The reaction conditions favour the formation of β-configurated C-nucleosides which renders additional epimerisation steps unnecessary. As a result, protected β-aryl-C-nucleosides are available directly from non-substituted arenes in three steps overall.

 Please wait while we load your content...
Please wait while we load your content...