C-Terminal properties are important for ring-fused 2-pyridones that interfere with the chaperone function in uropathogenic E. coli†
Abstract
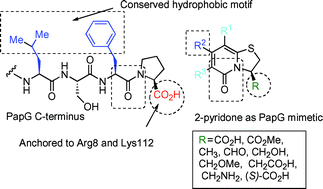
Virulence-associated organelles, termed pili or fimbriae, are assembled via the highly conserved chaperone–usher pathway in a vast number of pathogenic bacteria. Substituted bicyclic 2-pyridones, pilicides, inhibit pilus formation, possibly by interfering with the active site residues Arg8 and Lys112 of chaperones in uropathogenic E. coli. In this article we describe the synthesis and evaluation of nine analogues of a biologically active pilicide. Derivatization was performed with respect to its C-terminal features and the affinities for the chaperone PapD were studied with 1H relaxation-edited NMR spectroscopy. It could be concluded that the carboxylic acid functionality and also its spatial location was important for binding. In all cases, binding was significantly reduced or even abolished when the carboxylic acid was replaced by other substituents. In addition, in vivo results from a hemagglutination assay are presented where the derivatives have been evaluated for their ability to inhibit pilus formation in uropathogenic E. coli.

 Please wait while we load your content...
Please wait while we load your content...