Asymmetric syntheses of (−)-lentiginosine and an original pyrrolizidinic analogue thereof from a versatile epoxyamine intermediate
Abstract
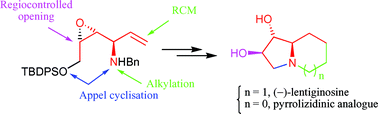
Ready access to natural (−)-lentiginosine and its pyrrolizidinic analogue from a chiral vinylic epoxyamine in a straightforward five-step sequence is presented. Careful use of the RCM reaction on aminotriols 5 and 6 constitutes the key feature of the synthetic pathway. The α-amyloglucosidase inhibitory activities of the target compounds were evaluated and showed that the more easily accessible pyrrolizidinic analogue possesses an inhibitory activity quite similar to that of (−)-lentiginosine.

 Please wait while we load your content...
Please wait while we load your content...