A highly stereoselective ether directed palladium catalysed aza-Claisen rearrangement†
Abstract
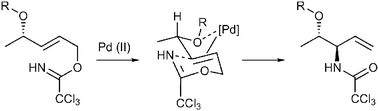
A highly stereoselective rearrangement of allylic trichloroacetimidates to allylic trichloroamides has been achieved using adjacent ether groups to direct facial coordination of the palladium(II)

 Please wait while we load your content...
Please wait while we load your content...