The facile preparation of primary and secondary aminesvia an improved Fukuyama–Mitsunobu procedure. Application to the synthesis of a lung-targeted gene delivery agent
Abstract
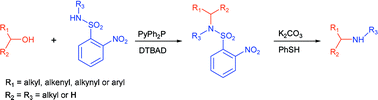
An efficient modification of the Fukuyama–Mitsunobu procedure has been developed whereby primary or

 Please wait while we load your content...
Please wait while we load your content...