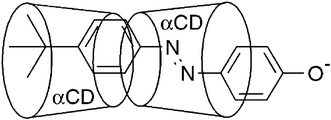
α-Cyclodextrin, β-cyclodextrin, N-(6A-deoxy-α-cyclodextrin-6A-yl)-N′-(6A-deoxy-β-cyclodextrin-6A-yl)urea and N,N-bis(6A-deoxy-β-cyclodextrin-6A-yl)urea (αCD, βCD, 1 and 2) form inclusion complexes with E-4-tert-butylphenyl-4′-oxyazobenzene, E-3−. In aqueous solution at pH 10.0, 298.2 K and I
= 0.10 mol dm−3
(NaClO4) spectrophotometric UV-visible studies yield the sequential formation constants: K11
=
(2.83 ± 0.28)
× 105 dm3 mol−1 for αCD·E-3−, K21
=
(6.93 ± 0.06)
× 103 dm3 mol−1 for (αCD)2·E-3−, K11
=
(1.24 ± 0.12)
× 105 dm3 mol−1 for βCD·E-3−, K21
=
(1.22 ± 0.06)
× 104 dm3 mol−1 for (βCD)2·E-3−, K11
=
(3.08 ± 0.03)
× 105 dm3 mol−1 for 1·E-3−, K11
=
(8.05 ± 0.63)
× 104 dm3 mol−1 for 2·E-3− and K12
=
(2.42 ± 0.53)
× 104 dm3 mol−1 for 2·(E-3−)2. 1H ROESY NMR studies show that complexation of E-3− in the annuli of αCD, βCD, 1 and 2 occurs. A variable-temperature 1H NMR study yields k(298 K)
= 6.7 ± 0.5 and 5.7 ± 0.5 s−1, ΔH‡
= 61.7 ± 2.7 and 88.1 ± 4.2 kJ mol−1 and ΔS‡
=
−22.2 ± 8.7 and 65 ± 13 J K−1 mol−1 for the interconversion of the dominant includomers (complexes with different orientations of αCD) of αCD·E-3− and (αCD)2·E-3−, respectively. The existence of E-3− as the sole isomer was investigated through an ab initio study.

 Please wait while we load your content...
Please wait while we load your content...