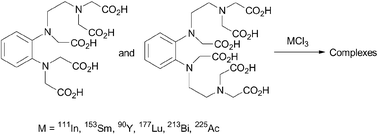
We wish to report the synthesis and metal complexation properties of new radionuclide chelating agents for use in nuclear medicine. The strategy includes the facile preparation of rigid analogues of DTPA and TTHA possessing an aromatic ring. The aromatic structure used increased the stability of the complexes formed (pre-organization concept) and they are easily functionalised for attaching to any support. The poly(amino)poly(carboxylic) acids, Ph-DTPA (5a) and Ph-TTHA (5b) were obtained in five steps from phenylenediamine as the starting material with overall yields of 42 and 20%, respectively. The key step in this synthetic process is the preparation of tri- and tetra-amino compounds, 3a and 3b, respectively. In order to assess the ability of both ligands to complex with different metals (111In, 153Sm, 90Y, 177Lu, 213Bi, 225Ac), along with their suitability for use in nuclear medicine, we used a number of complementary tests. We were able to demonstrate the high complexation capacity of Ph-DTPA (5a) with a broad range of radionuclides in a slightly acidic medium. In vitro stability studies show the high stability of Ph-DTPA with 111In in human serum, a necessary condition for all medical applications. The protonation constant (log KHi) of Ph-DTPA (5a) was determined by potentiometric methods.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...