Asymmetric cyclopropane synthesis via phosphine oxide mediated cascade reactions
Abstract
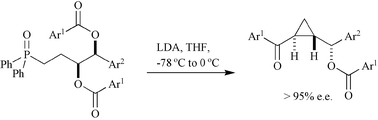
A silyloxy-THF has been converted into a cyclopropane containing three stereocentres as mixture of diastereoisomers. The mechanism of the reaction has been established and the source of stereochemical leakage proposed. An alternative stereospecific cascade reaction has been discovered.
- This article is part of the themed collection: In memory of Stuart Warren

 Please wait while we load your content...
Please wait while we load your content...