Barriers to internal rotation around the C–N bond in 3-(o-aryl)-5-methyl-rhodanines using NMR spectroscopy and computational studies. Electron density topological analysis of the transition states
Abstract
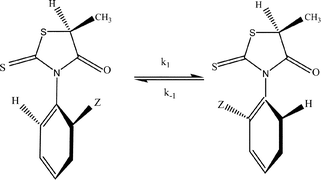
We have investigated the pairs of rotational isomers for six 3-(o-aryl)-5-methyl-rhodanines (Z = H, F, Cl, Br, OH, and CH3) using NMR spectroscopy and density functional theory (DFT) calculations. Electron density topological and NBO analysis has demonstrated the importance of non-covalent interactions, characterised by (3, −1) bond critical points (BCPs), between the oxygen and sulfur atoms on the thiazolidine ring with the aryl substitutents in stabilizing the transition states. The energetic activation barriers to rotation have also been determined using computational results; rotational barriers for 3-(o-chlorophenyl)-5-methyl-rhodanine (3S) and 3-(o-tolyl)-5-methyl-rhodanine (6S) were determined experimentally based on NMR separation of the diastereoisomeric pairs, and the first-order rate constants used to derive the value of the rotational barrier from the Eyring equation.

 Please wait while we load your content...
Please wait while we load your content...