A fluorine scan of the phenylamidinium needle of tricyclic thrombin inhibitors: effects of fluorine substitution on pKa and binding affinity and evidence for intermolecular C–F⋯CN interactions
Abstract
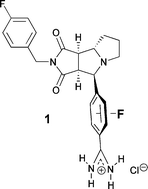
The H-atoms of the phenylamidinium needle of tricyclic

 Please wait while we load your content...
Please wait while we load your content...