Kinetics and mechanism of benzylamine additions to ethyl α-acetyl-β-phenylacrylates in acetonitrile
Abstract
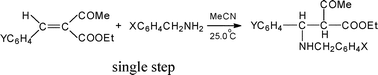
Kinetic studies of the addition of benzylamines to a noncyclic dicarbonyl group activated
The kinetic isotope effects (kH/kD > 1.0) measured with deuterated benzylamines (XC6H4CH2ND2) increase with a stronger electron acceptor substituent (δσX > 0) which is the same trend as those found for other dicarbonyl group activated series ( 1–4), but is in contrast to those for other ( noncarbonyl) group activated series (5–9). For the dicarbonyl series, the reactivity-selectivity principle (RSP) holds, but for others the anti-RSP applies. These are interpreted to indicate an insignificant imbalance for the former, but substantial lag in the resonance delocalization in the transition state for the latter series.

 Please wait while we load your content...
Please wait while we load your content...