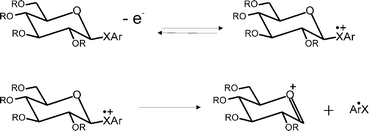
A series of six chalcoglycosides (phenyl-2,3,4,6-tetra-O-benzoyl-1-seleno-β-D-glucopyranoside, phenyl-2,3,4,6-tetra-O-benzyl-1-seleno-β-D-glucopyranoside, phenyl-2,3,4,6-tetra-O-benzyl-1-thio-β-D-glucopyranoside, p-tolyl-2,3,4,6-O-benzoyl-1-thio-β-D-glucopyranoside, p-tolyl-2,3,4,6-O-benzyl-1-thio-β-D-glucopyranoside, and phenyl-2,3,4,6-O-benzyl-β-D-glucopyranoside) are voltammetrically interrogated in dimethyl sulfoxide, so as to determine their formal (i.e. thermodynamic) redox potentials. The electrochemical oxidation of the chalcoglycoside is shown to follow an overall EC-type mechanism, in which the electro-generated cation radical undergoes an irreversible carbon–chalcogen bond rupture to produce the corresponding glycosyl cation, which may react further. The kinetics of the initial heterogeneous electron transfer process and subsequent irreversible homogeneous chemical degradation of the radical cation are reported, with values for the standard electrochemical rate constant k0 in the order of 10−2 cm s−1 and the first order homogeneous rate constant, k1, of the order of 103 s−1. The formal oxidation potentials were found to vary according to the identity of the chalcogenide, such that OPh > SPh ∼ STol > SePh.

 Please wait while we load your content...
Please wait while we load your content...