Substrate-assisted antibody catalysis
Abstract
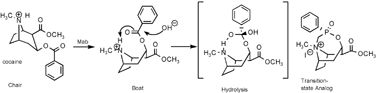
A new strategy in transition-state analog design is demonstrated to elicit catalytic antibodies. The strategy is based on substrate-assisted antibody catalysis and utilizes analogs designed to mimic the transition-state for intramolecular catalysis and thereby favor antibodies that can recruit catalytic groups from substrate. The

 Please wait while we load your content...
Please wait while we load your content...