Transition state stabilization and substrate strain in enzyme catalysis: ab initio QM/MM modelling of the chorismate mutase reaction
Abstract
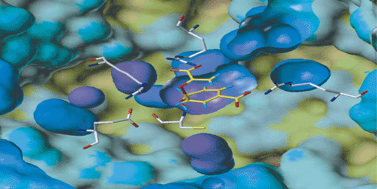
To investigate fundamental features of enzyme catalysis, there is a need for high-level calculations capable of modelling crucial, unstable species such as transition states as they are formed within enzymes. We have modelled an important model enzyme reaction, the Claisen rearrangement of chorismate to prephenate in chorismate mutase, by combined ab initio quantum mechanics/molecular mechanics (QM/MM) methods. The best estimates of the potential energy barrier in the enzyme are 7.4–11.0 kcal mol−1
(MP2/6-31+G(d)//6-31G(d)/CHARMM22) and 12.7–16.1 kcal mol−1
(B3LYP/6-311+G(2d,p)//6-31G(d)/CHARMM22), comparable to the experimental estimate of ΔH‡
= 12.7 ± 0.4 kcal mol−1. The results provide unequivocal evidence of transition state (TS) stabilization by the enzyme, with contributions from residues Arg90, Arg7, and Arg63. Glu78 stabilizes the prephenate product (relative to substrate), and can also stabilize the TS. Examination of the same pathway in solution (with a variety of continuum models), at the same ab initio levels, allows comparison of the catalyzed and uncatalyzed reactions. Calculated barriers in solution are 28.0 kcal mol−1
(MP2/6-31+G(d)/

 Please wait while we load your content...
Please wait while we load your content...