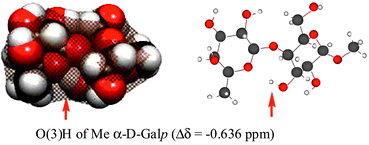
Density functional theory (DFT) and Hartree–Fock (HF) quantum mechanical calculations have been performed on the disaccharides, β-L-Fucp-(1→4)-α-D-Galp-OMe, β-L-Fucp-(1→4)-α-D-Glcp-OMe, and β-L-Fucp-(1→3)-α-D-Glcp-OMe. The Δδ-values (difference between the chemical shift in the disaccharide and the corresponding monosaccharide methyl glycoside) for the exchangeable hydroxy protons have been calculated and compared to experimental values previously measured by NMR spectroscopy for samples in aqueous solutions. The calculations performed on molecules in vacuum showed that hydroxy protons hydrogen bonded to the neighboring ring oxygens have large positive Δδ-values, indicating that they are deshielded relative to those in the corresponding methyl glycoside. The NMR experiments showed instead that these hydroxy protons close to the neighboring ring oxygens were shielded. This discrepancy between calculated and experimental data was attributed to solvent effects, and this hypothesis has been confirmed in this work by monitoring the chemical shift of the hydroxy proton of methanol in water, ethers and water/ether solutions. Shielding of the hydroxy proton of methanol is observed for increased ether concentrations, whereas deshielding is observed for increased concentration of water. The shielding observed for hydroxy protons in disaccharides is a consequence of reduced hydration due to intermolecular hydrogen bonding or steric effects. In strongly hydrated systems such as carbohydrates, the hydration state of a hydroxy proton is the key factor determining the value of the chemical shift of its NMR signal, and the Δδ will be a direct measure of the change in hydration state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...