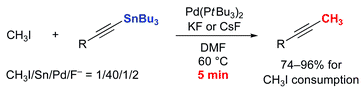
Rapid methylation of terminal acetylenes by the Stille coupling of methyl iodide with alkynyltributylstannanes: a general protocol potentially useful for the synthesis of short-lived 11CH3-labeled PET tracers with a 1-propynyl group†
Abstract
The Pd(0)-mediated rapid coupling (trapping) reaction of

 Please wait while we load your content...
Please wait while we load your content...