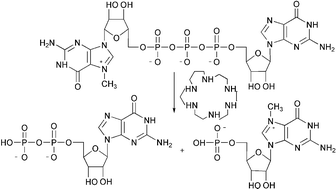
The reactions of a 5′-cap model compound P1-(7-methylguanosine)
P3-guanosine 5′,5′-triphosphate, m7GpppG, were studied in the presence of three different macrocyclic amines (2–4) under neutral conditions. The only products observed in the absence of the macrocycles resulted from the base-catalysed imidazole ring-opening and the acid-catalysed cleavage of the N7-methylguanosine base, whereas in the presence of these catalysts hydrolysis of the triphosphate bridge predominated. The latter reaction yielded guanosine 5′-monophosphate, guanosine 5′-diphosphate, 7-methylguanosine 5′-monophosphate and 7-methylguanosine 5′-diphosphate as the initial products, indicating that both of the phosphoric anhydride bonds were cleaved. The overall catalytic activity of all three macrocycles was comparable. The hydrolysis to guanosine 5′-diphosphate and 7-methylguanosine 5′-monophosphate was slightly more favoured than the cleavage to yield guanosine 5′-monophosphate and 7-methylguanosine diphosphate. All the macrocycles also enhanced the subsequent hydrolysis of the nucleoside diphosphates, 2 being more efficient than 3 and 4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...