Stereocontrol of 1,5-related stereocentres using an intermediate silyl group—the diastereoselectivity of nucleophilic attack on a double bond adjacent to a stereogenic centre carrying a silyl group
Abstract
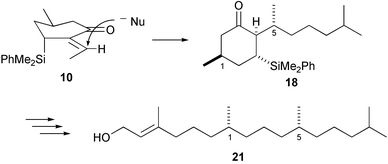
R-5-Methylcyclohex-2-enone 1 reacts successively with the phenyldimethylsilylzincate reagent and acetaldehyde to give with regiocontrol the aldols 7, dehydration of which creates the E-exocyclic double bond of the α,β-unsaturated ketone 2. Conjugate addition of the ethylcuprate reagent to this compound takes place with high (96 ∶ 4) selectivity in favour of the R stereoisomer 12, hydrolysis of which gives (2R,3R,5S,2′R)-2-(but-2′-yl)-3-dimethyl(phenyl)silyl-5-methylcyclohexanone 3. The oxime acetate of this ketone undergoes fragmentation in the presence of trimethylsilyl trifluoromethanesulfonate to give 3R,7R,5E-3,7-dimethylnon-5-enonitrile 4, in which an open-chain 1,5-stereochemical relationship is set up with a high level of stereocontrol. A similar sequence adding 4-methylpentylcuprate to the enone 2, and fragmentation gives 3R,7R,5E-3,7,11-trimethyldodec-5-enonitrile 20. Reduction and hydrogenation of this nitrile gives 3R,7R-3,7,11-trimethyldodecanal 22, which can be converted into phytol 25. The ketoaldehyde 29 reacts with samarium iodide to give only the alcohol 30, in which the radical anion has attacked from the top surface, just like the cuprate reagents in their reactions with the ketone 2.

 Please wait while we load your content...
Please wait while we load your content...