Asymmetric alkene epoxidation catalysed by a novel family of chiral metalloporphyrins: effect of structure on catalyst activity, stability and enantioselectivity
Abstract
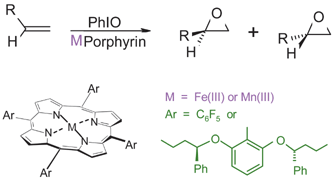
A selection of alkenes has been epoxidised with iodosylbenzene, catalysed by three related iron(III) tetraarylporphyrins: 1*, 2* and 3* with four 2,6-di(1-phenylbutoxy)phenyl groups, with one pentafluorophenyl and three 2,6-di(1-phenylbutoxy)phenyl groups and with two pentafluorophenyl and two 2,6-di(1-phenylbutoxy)phenyl groups, respectively. 1* is very sterically hindered and prone to self-oxidation which makes it a relatively poor epoxidation catalyst. Introducing the smaller pentafluorophenyl groups, in place of 2,6-di(1-phenylbutoxy)phenyl, increases catalyst reactivity, stability and selectivity. This change allows easier access of the substrates to the active oxidant and also, by decreasing the electron density on the porphyrin ligand, increases the reactivity of the oxoiron intermediate and its stability towards self-oxidation. A family of five homochiral catalysts, 1, 2 and 3, [the analogues of 1*, 2* and 3*, prepared from (R,R)-2,6-di(1-phenylbutoxy)benzaldehyde] and catalyst 4 with three pentafluorophenyl and one (R,R)-2,6-di(1-phenylbutoxy)phenyl group and 5 the manganese(III) analogue of 3 have been used to epoxidise three prochiral alkenes. All the reactions give low enantioselectivities. Using styrene as the substrate, (S)-styrene epoxide is the major enantiomer obtained with all the catalysts except 1 which leads to the (R)-styrene epoxide being preferred. In contrast cis-hept-2-ene and 2-methylbut-2-ene give the same major epoxide enantiomer with all the catalysts. The dependence of the ee values on catalyst and substrate structure, temperature and solvent is examined and discussed.

 Please wait while we load your content...
Please wait while we load your content...