Ketene-forming eliminations from aryl bis(4′-chlorophenyl)acetates promoted by R2NH–R2NH2+ in aqueous MeCN. Change of mechanism†
Abstract
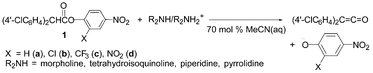
Elimination reactions of (4′-ClC6H4)2CHCO2C6H3-2-X-4-NO2 promoted by R2NH–R2NH2+ in 70 mol% MeCN (aq.) have been studied kinetically. The reactions are second-order and exhibit Brönsted β = 0.44–0.86 and |βlg| = 0.41–0.71. The Brönsted β decreased with a poorer leaving group and |βlg| increased with a weaker base. The results are consistent with an E2 mechanism. When X = H, the reaction proceeded by the concurrent E2 and E1cb mechanims.

 Please wait while we load your content...
Please wait while we load your content...