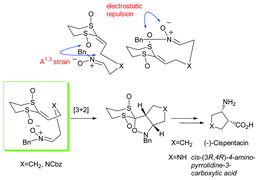
The use of enantiomerically pure ketene dithioacetal bis(sulfoxides) in highly diastereoselective intramolecular nitrone cycloadditions. Application in the total synthesis of the β-amino acid (−)-cispentacin and the first asymmetric synthesis of cis-(3R,4R)-4-amino-pyrrolidine-3-carboxylic acid
Abstract
Intramolecular 1,3-dipolar nitrone

 Please wait while we load your content...
Please wait while we load your content...