Chiral base-mediated benzylic functionalisation of tricarbonylchromium(0) complexes of benzylamine derivatives
Abstract
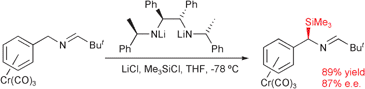
The novel complex (η6-benzylamine)tricarbonylchromium(0) 11 was prepared in up to 66% yield by direct complexation with Cr(CO)6 in refluxing 1,4-dioxane. Imine derivatives of this complex were readily deprotonated at the benzylic position by diamide 5, and the resultant anions reacted regioselectively with electrophiles (Me3SiCl or MeI) to give fairly good yields of products substituted at the benzylic carbon. Products of up to 87% e.e. were obtained in these reactions, with the highest enantioselectivity being derived from the tert-butyl-substituted imine complex 25.

 Please wait while we load your content...
Please wait while we load your content...