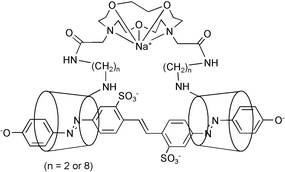
Complexation of the Brilliant Yellow tetraanion, 34−, by two new diazacoronand linked β-cyclodextrin (βCD) dimers 4,13-bis(2-(6A-deoxy-β-cyclodextrin-6A-yl)aminoethylamidomethyl- and 4,13-bis(8-(6A-deoxy-β-cyclodextrin-6A-yl)aminooctylamidomethyl)-4,13-diaza-1,7,10-trioxacyclopentadecane, 1 and 2, respectively, has been studied in aqueous solution. UV-visible spectrophotometric studies at 298.2 K, pH 10.0 and I
= 0.10 mol dm−3
(NEt4ClO4) yielded complexation constants for the complexes 1·34− and 2·34−, K1
=
(1.08 ± 0.01)
× 105 and (6.21 ± 0.08)
× 103 dm3 mol−1, respectively. Similar studies at 298.2 K, pH 10.0 and I
= 0.10 mol dm−3
(NaClO4) yielded K3
=
(4.63 ± 0.09)
× 105 and (3.38 ± 0.05)
× 104 dm3 mol−1 for the complexation of 34− by Na+·1 and Na+·2 to give Na+·1·34− and Na+·2·34−, respectively. Potentiometric studies of the complexation of Na+ by 1 and 2 by the diazacoronand component of the linkers to give Na+·1 and Na+·2 yielded K2
=
(2.00 ± 0.05)
× 102 and (1.8 ± 0.05)
× 103 dm3 mol−1, respectively, at 298.2 K and I
= 0.10 mol dm−3
(NEt4ClO4). For complexation of Na+ by 1·34− and 2·34− to give Na+·1·34− and Na+·2·34−K2K3/K1
=
K4
= 8.6 × 102 and 9.8 × 103 dm3 mol−1, respectively. The pKas of 1H44+ are 7.63 ± 0.01, 6.84 ± 0.02, 5.51 ± 0.04 and 4.98 ± 0.03, and those of 2H44+ are 8.67 ± 0.02, 8.11 ± 0.02, 6.06 ± 0.02 and 5.14 ± 0.05. The larger magnitude of K1 for 1 by comparison with K1 for 2 is attributed to the octamethylene linkers of 2 competing with 34− for occupancy of the annuli of the βCD entities while the competitive ability of the dimethylene linkers of 1 is less. A similar argument applies to the relative magnitudes of K3 for Na+·1 and Na+·2. Increased electrostatic attraction probably accounts for K3 > K1 for Na+·1·34− and 1·34− and for Na+·2·34− and 2·34−. The lesser magnitudes of K2 and K4 for Na+·1 and Na+·1·34− compared with those for Na+·2 and Na+·2·34− are attributed to the octamethylene linkers of 2 producing a more hydrophobic environment for the diazacoronand than that produced by the dimethylene linkers of 1. 1H NMR spectroscopic studies and the syntheses of 1 and 2 are described.

 Please wait while we load your content...
Please wait while we load your content...