Self-recognition and hydrogen bonding by polycyclic bridgehead monoalcohols†
Abstract
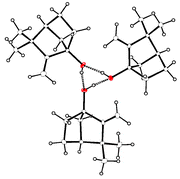
Our interest in the relationship between the hydrogen bonding motifs displayed by monoalcohols and the properties of the solids which contain these motifs has led us to determine the crystal structures of three polycyclic bridgehead monoalcohols. One C10H16O isomer crystallises in the space group P212121 but the three molecules which comprise the asymmetric unit are related approximately by the operations of a 31 screw axis. They are linked by hydrogen bonds to form an infinite helix. A second C10H16O isomer forms rings containing four molecules joined by cooperative hydrogen bonds. The chiral space group P41212 accommodates molecules of the S,S and R,R enantiomers in the molar ratio 92 ∶ 8 (ee 84%) owing to disorder. A related C9H14O2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding. These hydrogen bond motifs are shown to be typical for 45 tertiary monoalcohols, CmHnOH, present in the Cambridge Structural Database. Tertiary monoalcohols display in a more pronounced form the strong preferences for trigonal and tetragonal space groups and for asymmetric units containing several molecules which are established features of the crystallochemistry of monoalcohols.
C hydrogen bonding. These hydrogen bond motifs are shown to be typical for 45 tertiary monoalcohols, CmHnOH, present in the Cambridge Structural Database. Tertiary monoalcohols display in a more pronounced form the strong preferences for trigonal and tetragonal space groups and for asymmetric units containing several molecules which are established features of the crystallochemistry of monoalcohols.

 Please wait while we load your content...
Please wait while we load your content...