Antitumor benzothiazoles.† Frontier molecular orbital analysis predicts bioactivation of 2-(4-aminophenyl)benzothiazoles to reactive intermediates by cytochrome P4501A1
Abstract
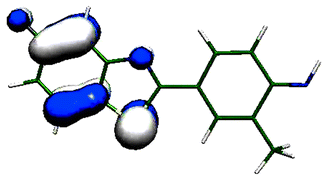
The antitumor and metabolic activities of 2-(4-aminophenyl)benzothiazoles and their fluorinated analogues cannot be explained or predicted by conventional chemical means. Their mode of anti-cancer action involves metabolism of the

 Please wait while we load your content...
Please wait while we load your content...