Intercalation–deintercalation of water-in-salt electrolytes in nanoscale hydrophobic confinement†
Abstract
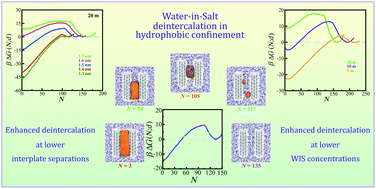
Intercalation–deintercalation of water-in-salt (WIS) electrolytes in nanoscale confinement is an important phenomenon relevant to energy storage and self-assembly applications. In this article, we use molecular simulations to investigate the effects of intersurface separation on the structure and free energy underlying the intercalation–deintercalation of the Li bis(trifluoromethane)sulfonimide ([Li][TFSI]) water-in-salt (WIS) electrolyte confined between nanoscale hydrophobic surfaces. We employ enhanced sampling to estimate the free energy profiles for the intercalation behaviour of WIS in confining sheets at several intersurface separations. We observe that the relative stability of the condensed and vapour phases of WIS in the confinement depends on the separation between the confining surfaces and the WIS concentration. We find that the critical separation at which the condensed and vapour phases are equally stable in confinement depends on the concentration of WIS. The relative height of the free energy barrier also strongly depends on the concentration of [Li][TFSI] inside the confined space, and we find that this concentration dependence can be attributed to changes in line tension. The process of deintercalation passes through vapour tube formation inside the confined space, and this process is initiated by vapour bubble formation. The size of the critical vapour tube required for spontaneous evaporation of WIS from the confinement is also found to depend on the intersurface separation and WIS concentration.


 Please wait while we load your content...
Please wait while we load your content...