Copper(i)–alkylamine mediated synthesis of copper nanowires†
Abstract
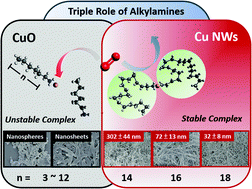
Formation of a Cu(I)–alkylamine complex is found to be the key step for Cu(II) ions to reduce to Cu(0) in the presence of glucose. Also, alkylamines in Cu nanowire synthesis serve triple roles as a reducing, complexation and capping agent. Alkylamines reduce Cu(II) to Cu(I) at above 100 °C and protect the Cu(I) by forming a Cu ion–alkylamine coordination complex with a 1 : 2 ratio in an aqueous solution. With respect to the 1 : 2 complex ratio, the additional free alkylamines ensure a stable Cu(I)–alkylamine complex. After completion of Cu(I)–Cu(0) reduction by glucose, alkylamines remain on Cu(0) seeds to regulate the anisotropic growth of Cu nanocrystals. Long-chain (≥C16) alkylamines are found to help produce high-quality Cu nanowires, while short-chain (≤C12) alkylamines only produce CuO products. Furthermore, Cu nanowire synthesis is found to be sensitive to additional chemicals as they may destabilize Cu ion–alkylamine complexes. By comparing the Cu(I)–alkylamine and Maillard reaction mediated mechanism, the complete Cu nanowire synthesis process using glucose is revealed.

 Please wait while we load your content...
Please wait while we load your content...