CoMo-bimetallic N-doped porous carbon materials embedded with highly dispersed Pt nanoparticles as pH-universal hydrogen evolution reaction electrocatalysts†
Abstract
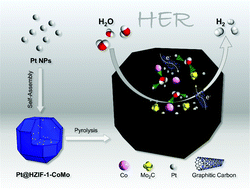
The hydrogen evolution reaction is a key half reaction for water electrolysis and is of great significance. Pt-based nanomaterials are promising candidates for HER electrocatalysts. However, the high price of platinum and poor durability impede their practical applications. Herein, a new CoMo-bimetallic hybrid zeolite imidazolate framework is employed to load Pt nanoparticles in a highly dispersed manner as a precursor to synthesize an efficient pH-universal HER electrocatalyst (PtCoMo@NC), which displays overpotentials of 26, 51, and 66 mV at a current density of 10 mA cm−2 in acidic, basic, and neutral media, respectively. The strong synergistic effect of highly dispersed multi-type catalytic species, including cobalt, molybdenum carbide, and platinum (4.7%) promotes the catalytic activity in the HER process. Meanwhile, the aggregation of Pt nanoparticles is greatly restrained by the carbon matrix so that a brilliant long-time durability of 12 hours and a negligible current decrease in the LSV curve after 10 000 CV cycles are achieved.


 Please wait while we load your content...
Please wait while we load your content...