Plasmonic thermochromism based on a reversible redox reaction of Ag+/Ag on Au nanorods†
Abstract
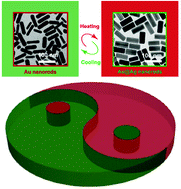
Reversible redox reaction-based thermochromism using plasmonic nanocrystals is challenging due to the requirements set based on the complexity of the reaction system where the oxidizing and reducing agents must not interfere with each other, and both should possess temperature sensitivity. Herein, we demonstrate plasmonic thermochromism based on a reversible redox reaction of Ag+/Ag on Au nanorods (AuNRs) by incorporating temperature-sensitive reducing and oxidizing agents into the same system. The competition between reduction and oxidation is solely dependent on temperature. When the temperature is above (below) the transition temperature, the reduction of Ag+ (oxidation of Ag) dominates on the surface of AuNRs, and the thermochromic nanostructure solution appears green (red). An experimental study on the mechanism reveals that HOCl produced at low concentrations by H2O2 is the source of the observed temperature dependence of the Ag oxidation. Rationally tuning the transition temperature in a range from 27 to 40 °C can be realized by changing the concentration of some key chemical compounds in the solution. The thermochromic solution can be standalone-functional within multiple cycles of heating and cooling and long-term storage without any additional reagents. Our study provides new insight into plasmonic thermochromism and may pave the way for fabricating smart thermochromic materials.


 Please wait while we load your content...
Please wait while we load your content...