Engineering interfacial structures to accelerate hydrogen evolution efficiency of MoS2 over a wide pH range†
Abstract
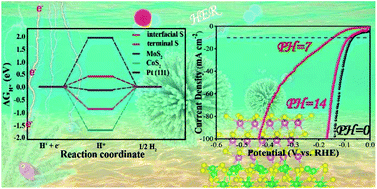
Developing low-cost electrocatalysts with outstanding electrochemical performance for water splitting over a wide pH range is urgently desired to meet the practical needs in different areas. Herein, a highly efficient hierarchical flower-like CoS2@MoS2 core–shell nanostructured electrocatalyst is fabricated by a two-step strategy, in which MoS2 nanosheets with a layered structure are grown on the CoS2 core supported on carbon paper (CP) and used as hydrogen evolution reaction (HER) electrocatalysts working in the whole pH range (0–14). Remarkably, benefiting from the interface-engineering in this 3D core–shell structure of the electrocatalyst, the optimum CoS2@MoS2/CP catalyst exhibits outstanding HER activity over a wide range of pH values and an overpotential of 69 mV in acidic solution, 145 mV in neutral solution and 82 mV in alkaline solution, respectively, to afford the standard current density of 10 mA cm−2. Furthermore, it demonstrates superior stability under different pH conditions for at least 48 h. Density functional theory (DFT) calculations are performed to gain further insight into the effect of CoS2@MoS2 interfaces, revealing that the strong interfacial interaction between CoS2 and MoS2 dramatically reduces the Gibbs free energy of hydrogen adsorption and the energy barrier for water dissociation, thus enhancing the electrochemical HER activity in the whole pH range (0–14).


 Please wait while we load your content...
Please wait while we load your content...