Tunable morphologies and emission of photosensitive supramolecular self-assemblies through positional and trans–cis isomerization†
Abstract
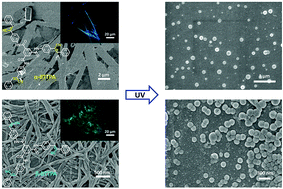
Cyanostilbene units are widely attractive as photoresponsive supramolecular building blocks whose structures and emission can be modulated by trans–cis isomerization. Generally, the change of properties is related to the molecular structure of cyanostilbene, which is still unpredictable and needs to be explored. Herein, two benzene-1,3,5-tricarboxamide (BTA) based cyanostilbene derivatives with different cyano positions have been designed to investigate the emission as well as structural changes during the trans–cis photoisomerization process in monomer and aggregation states, respectively. In the monomer state, the derivative with cyano groups at the outer position, β-BTTPA, exhibits obvious emission enhancement upon UV irradiation, while the other derivative (α-BTTPA) shows emission quenching. In addition, upon the formation of aggregates, β-BTTPA forms nano-level fibers with blue-green emission, but α-BTTPA forms micron-level flat ribbons with blue emission. More importantly, also driven by the trans–cis photoisomerization, the self-assemblies show morphological transitions (ribbons/fibers to spheres) due to the fact that the equilibrium of the system is broken by the photoreactions. Such changes further contribute to emission switching as well as enhanced hydrophobic properties.


 Please wait while we load your content...
Please wait while we load your content...