Covalent organic framework-supported platinum nanoparticles as efficient electrocatalysts for water reduction†
Abstract
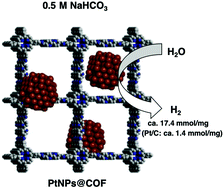
The hydrogen evolution reaction (HER) is one of the most effective and sustainable ways to produce hydrogen gas as an alternative clean fuel. The rate of this electrocatalytic reaction is highly dependent on the properties (dispersity and stability) of electrocatalysts. Herein, we developed well-dispersed and highly stable platinum nanoparticles (PtNPs) supported on a covalent organic framework (COF-bpyTPP), which exhibit excellent catalytic activities toward HER as well as the hydride reduction reaction. The nanoparticles have an average size of 2.95 nm and show superior catalytic performance compared to the commercially available Pt/C under the same alkaline conditions, producing 13 times more hydrogen with a far more positive onset potential (−0.13 V vs. −0.63 V) and ca. 100% faradaic efficiency. The reaction rate of the hydride reduction of 4-nitrophenol was also 10 times faster in the case of PtNPs@COF compared to the commercial Pt/C under the same loading and conditions. More importantly, the PtNPs@COF are highly stable under the aqueous reactions conditions and can be reused without showing noticeable aggregation and activity degradation.


 Please wait while we load your content...
Please wait while we load your content...