Revisiting the positive roles of liquid polysulfides in alkali metal–sulfur electrochemistry: from electrolyte additives to active catholyte
Abstract
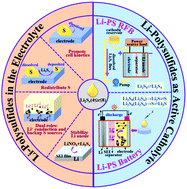
Polysulfide dissolution and shuttling in liquid organic electrolytes are considered as the most challenging detrimental effects of an Li–S cell, which is one of the most promising next-generation high-energy-density batteries. Therefore, considerable efforts have been devoted to confining solid sulfur or sulfide so as to avoid the formation and diffusion of dissolved polysulfides. However, the positive roles played by the liquid polysulfides in Li–S electrochemistry should not be overlooked. Polysulfide dissolution can promote the cell kinetics and sulfur utilization; as electrolyte additives, polysulfides can help stabilize the Li metal anode, redistribute the active mass in the cathode and act as extra back-up active sulfur sources. After being applied directly as active catholytes, a novel Li-polysulfide redox flow battery (Li-PS RFB) and an Li-polysulfide battery (Li-PS battery) have been developed. This review revisited these beneficial effects of polysulfides and provided a summary of the recent progress on Li-PS RFB and Li-PS batteries, especially with a more comprehensive emphasis on the latter. Furthermore, dissolved polysulfides applied as active catholytes in Na–S and K–S systems and as catholytes or anolytes in aqueous batteries were also briefly discussed. Hopefully, the Li–S electrochemistry can be better understood so as to overcome challenging issues in the way of the practical commercialization of the Li–S batteries.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...