Halide-assisted metal ion reduction: emergent effects of dilute chloride, bromide, and iodide in nanoparticle synthesis†
Abstract
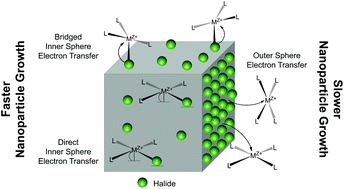
Understanding the competing effects of growth-directing additives, such as halide ions, on particle formation in solution phase metal nanoparticle syntheses is an ongoing challenge. Further, trace halide impurities are known to have a drastic impact on particle morphology as well as reproducibility. Herein, we employ a “halide-free” platform as an analogue to commonly used halide-containing surfactants and metal precursors to isolate and study the effects of micromolar concentrations of halide ions (chloride, bromide, and iodide) on the rate of metal ion reduction. In the absence of competing halides from precursors and surfactants, we observe a catalytic effect of low concentrations of halide ions on the rate of metal ion reduction, an influence which is fundamentally different from the previously reported role of halides in metal nanoparticle growth. We propose that this halide-assisted metal ion reduction proceeds via the formation of a halide bridge which facilitates the adsorption of the metal precursor to a growing nanoparticle and, subsequently, electron transfer from the particle surface. We then demonstrate that this process is operative not only in the well-controlled “halide-free” platform, but also in syntheses involving high concentrations of halide-containing surfactants as well as metal precursors with halide ligands. Importantly, this study shows that halide-assisted metal ion reduction can be extended to bimetallic systems and provides a handle for the directed differential control of metal ion reduction in one-pot co-reduction syntheses.


 Please wait while we load your content...
Please wait while we load your content...
