Regulating the electron density of dual transition metal sulfide heterostructures for highly efficient hydrogen evolution in alkaline electrolytes†
Abstract
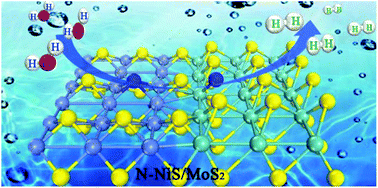
Exploring highly effective electrocatalysts with heterostructures is significant for sustainable hydrogen production by the hydrogen evolution reaction (HER). However, there are still challenges in improving the HER activities of the heterostructures to achieve efficient hydrogen production. Here, nitrogen-decorated dual transition metal sulphide heterostructures (N-NiS/MoS2) were constructed with an enhanced HER performance in alkaline electrolytes. These novel N-NiS/MoS2 heterostructures exhibited a low overpotential of 71 mV (10 mA cm−2), small Tafel slope of 79 mV dec−1 and favorable stability. In particular, the experimental and theoretical calculation results consistently demonstrated that the introduction of nitrogen can effectively tune the electronic structure of the heterostructures. Furthermore, the synergistic effect between dual-active components N-NiS and N-MoS2 in the N-NiS/MoS2 heterostructures effectively promoted water dissociation and hydrogen formation, leading to remarkable increase in the HER performance in an alkaline medium. This work provides a valuable avenue for the rational modulation of the electronic structure of heterostructures by hetero-atoms for highly efficient HER catalysts.


 Please wait while we load your content...
Please wait while we load your content...