The molecular basis of interaction domains of full-length PrP with lipid membranes†
Abstract
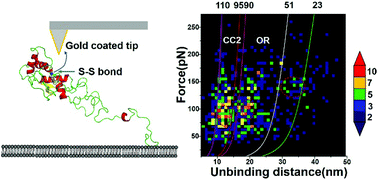
PrP–lipid membrane interactions are critical to PrP structural conversion and neurotoxicity, but its molecular mechanism remains unclear. A two-dimensional histogram of force–distance curves and a worm-like chain model revealed three binding regions at the PrP N-terminal, providing the molecular basis for understanding the interactions between full-length PrP and lipid membranes.


 Please wait while we load your content...
Please wait while we load your content...
