All-inorganic lead halide perovskite nanohexagons for high performance air-stable lithium batteries†
Abstract
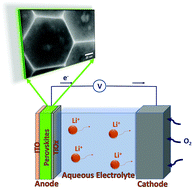
All-inorganic Cs4PbBr6 perovskite nanohexagons, pre-synthesized by a room temperature co-precipitation method, have been electrochemically investigated in a conventional aqueous electrolyte for potential application as an anode material in Li-ion batteries. The nanohexagons were uniformly deposited on ITO precoated glass substrate and subsequently annealed at ambient air to form a mechanically stable perovskite layer. These perovskite layers showed excellent performance during continuous Li-ion intercalation/deintercalation scans in an aqueous electrolyte, exhibiting a diffusion coefficient of 7.34 × 10−8 cm2 s−1, a specific discharge capacity of 377 mA h g−1, a capacity retention of 75% and coulombic efficiency that deteriorated to 98% after 100 scans. A water-triggered transformation of the Cs4PbBr6 to the CsPb2Br5 was initially observed followed by a reversible Li intercalation/deintercalation in the CsPb2Br5 structure for 40 consecutive scans. Following this period, an irreversible conversion reaction of CsPb2Br5 to CsBr and PbBr2 took place. The excellent electrochemical performance observed is promising towards the potential application of all-inorganic perovskite nanocrystals for air-stable, lithium storage applications.


 Please wait while we load your content...
Please wait while we load your content...