Highly coke resistant Mg–Ni/Al2O3 catalyst prepared via a novel magnesiothermic reduction for methane reforming catalysis with CO2: the unique role of Al–Ni intermetallics†
Abstract
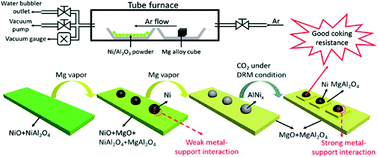
Addition of alkaline promoters is considered to be an effective way to improve the coking resistance of the metal/support composite catalysts for dry reforming of methane (DRM). The traditional metal/promoter/support composites for DRM catalysis are generally obtained from alkaline species impregnation and then high temperature H2 reduction. This two-step process leads to a random distribution of metal–promoter interaction. We herein report a novel magnesiothermic method to reduce Ni from spinel precursor and introduce alkaline Mg(II) into the composite at the same time, which also gratifies the interaction between the promoter and metal nanoparticles (NPs). The reaction paths to Mg reduction are proposed. The as prepared catalysts show good activity and outstanding coking resistance in DRM. The Ni–Al intermetallics in the catalyst were found for the first time to play an important role in coking resistance as they can be in situ transformed into Ni nanoparticles and MgAl2O4 with strong metal–support interaction during the DRM.


 Please wait while we load your content...
Please wait while we load your content...