Charge transfer dynamics in CsPbBr3 perovskite quantum dots–anthraquinone/fullerene (C60) hybrids†
Abstract
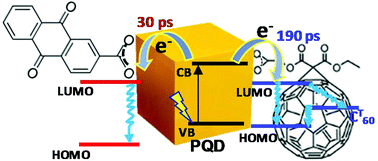
An advantage of colloidal quantum dots, particularly perovskite quantum dots (PQDs), as photoactive components is that they easily form complexes with functional organic molecules, which results in hybrids with enriched photophysical properties. Herein, we demonstrate the formation of stable ground state complexes of CsPbBr3 PQD with two widely used molecular electron acceptors, fullerene (C60) and anthraquinone, (AQ) which contain carboxylic anchor groups. Dynamics of the photo-induced electron transfer in the hybrids were compared. The use of carboxylic groups for binding results in stable complex formation and their photophysical properties depend on the ratio of components but not the absolute concentrations (up to micromolar concentrations). Time-resolved transient absorption (TA) spectroscopy shows that in both cases, a charge separated (CS) state is formed. Data analysis was aimed to evaluate the CS time constant in ideal one-to-one complexes and was found to be in the range of 30–190 ps. The CS state of PQD–AQ complexes recombines directly to the ground state in roughly one microsecond. Recombination of the CS state of PQD–C60 is more complex and points to strong inhomogeneity of these complexes. Majority of the CS states relax by first forming the C60 triplet state.


 Please wait while we load your content...
Please wait while we load your content...