Nanographene favors electronic interactions with an electron acceptor rather than an electron donor in a planar fused push–pull conjugate†
Abstract
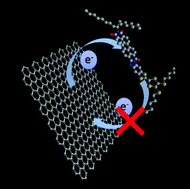
A combination of a preexfoliated nanographene (NG) dispersion and fused electron donor–acceptor tetrathiafulvalene–perylenediimide (TTF–PDI) results in a noncovalent functionalization of NG. Such novel types of nanohybrids were characterized by complementary spectroscopic and microscopic techniques. The design strategy of the chromophoric and electroactive molecular conjugate renders a large and planar π-extended system with a distinct localization of electron-rich and electron-poor parts at either end of the molecular conjugate. Within the in situ formed nanohybrid, the conjugate was found to couple electronically with NG preferentially through the electron accepting PDI rather than the electron donating TTF and to form the one-electron reduced form of PDI, which corresponds to p-doping of graphene.


 Please wait while we load your content...
Please wait while we load your content...