A self-encapsulated porous Sb–C nanocomposite anode with excellent Na-ion storage performance†
Abstract
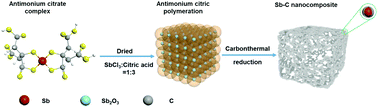
In this study, a self-encapsulated Sb–C nanocomposite as an anode material for sodium-ion batteries (SIBs) was successfully synthesised using an SbCl3–citrate complex precursor, followed by a drying and calcination process under an inert N2 atmosphere. When the molar ratio of SbCl3 to citric acid was varied from 1 : 1 to 1 : 4, the Sb–C nanocomposite with a molar ratio of 1 : 3 (Sb–C3) exhibited the highest specific surface area (265.97 m2 g−1) and pore volume (0.158 cm3 g−1). Furthermore, the Sb–C3 electrode showed a high reversible capacity of 559 mA h g−1 at a rate of C/10 and maintained a high reversible capacity of 430 mA h g−1 even after 195 cycles at a rate of 1C. The Sb–C3 electrode exhibited an excellent rate capability of 603, 445, and 357 mA h g−1 at the rates of C/20, 5C, and 10C, respectively. Furthermore, a full cell composed of an Sb–C3 anode and a Na3V2(PO4)3 cathode exhibited good specific capacity and cyclability, making the Sb–C composite a promising anode material for high-performance SIBs.


 Please wait while we load your content...
Please wait while we load your content...