Fast naked-eye detection of zinc ions by molecular assembly-assisted polymerization of diacetylene†
Abstract
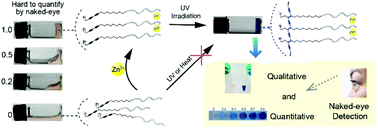
Portable visual detection systems for environmental monitoring or diagnostic purposes are eagerly anticipated in low-resource settings. Inexpensive device requirements and visualization are key challenges for the development of any portable analysis system. We report herein a new strategy for developing portable rapid ion detection technology by the coupling of topochemical polymerization and supramolecular (SM) self-assembly. The rapid sol–gel or gel–sol phase transition of SM hydrogels has been widely applied for the detection of many important analytes including metal ions. However, one problem that remains is the difficulty inaccurately quantifying the degree of self-assembly with the naked eye. To address this problem, we designed a diacetylene-grafted peptide that can be polymerized following self-assembly into a hydrogel triggered by zinc ions. Before adding zinc ions, the molecules dissolved well in aqueous solution and arranged randomly, and were unable to be polymerized through UV light irradiation. After mixing with zinc ions, the peptide chelated with zinc ions immediately and self-assembled into a SM hydrogel. The molecules arranged orderly and could be easily polymerized through irradiation of a hand-held UV lamp in less than 2 minutes. The hydrogel showed a quick and sharp chromatic change from colorless to dark blue, which allowed the quantification of self-assembly (i.e. concentration of zinc ions) with the naked eye. In addition, the monomers were insensitive to light, pH and temperature changes, which is a highly desired characteristic in practical applications.


 Please wait while we load your content...
Please wait while we load your content...